ADAM15 is a “sheddase” known to promote pathological angiogenesis in models of ocular neovascular disease and whose expression correlates strongly with the progression of several aggressive adenocarcinomas (1-5). ADAM15 null mice are viable and display no major abnormalities during adult life. However, these mice showed a severely attenuated pathological angiogenic response in several models recapitulating ocular neovascular diseases and tumours were also observed to grow more slowly when human tumour cell lines were implanted (6, 7). Researchers also demonstrated that ADAM15 and VEGF participate in an amplification loop in the eye as the expression of VEGF and its receptors were reduced in ADAM15 null retinas. Conversely, ADAM15 was highly up regulated (up to 50-fold) by both ischemia and VEGF overexpression in transgenic mice, thereby identifying a pathological synergy between these angiogenic mediators (7)
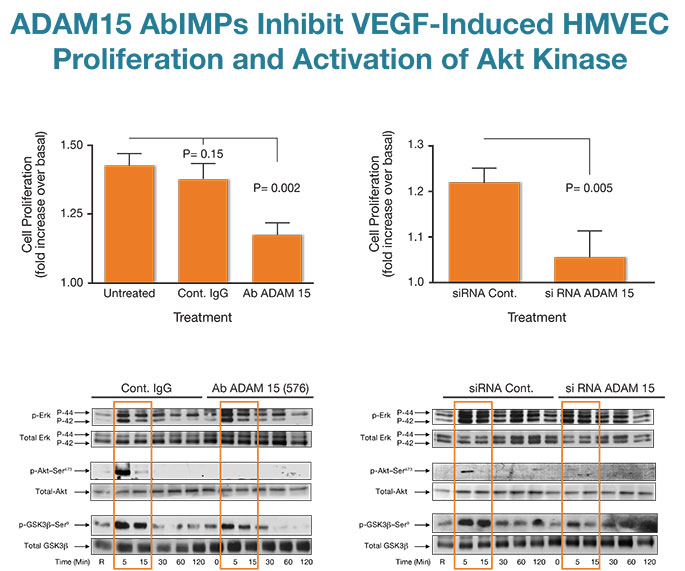
Fig. 3
Applying novel AbIMP™ technology to study the role of ADAM15 in angiogenesis and endothelial cell biology, we showed that ADAM15 AbIMPs dysregulated the morphogenesis of vessels in the murine post-natal retina by impairing endothelial cell proliferation in vessel stalks without effecting tip cell numbers needed for new sprouts (ref. 8 & 9 and unpublished data). ADAM15 AbIMPs also inhibited the proliferation of cultured human endothelial cells (HMVECs) in response to VEGF and these results were recapitulated by knockdown of the entire molecule with selective siRNA (Fig. 3, top panel). Both ADAM15 AbIMPs and siRNA inhibited VEGF-induced activation of the important growth and survival promoting kinase AKT and its downstream effector GSK3β but had no effect on the activation of the Erk/MAP kinases (Fig. 3, bottom panel).
These studies showed that ADAM15 is an important promoter of angiogenesis and VEGF proliferative and survival signals in endothelial cells. Importantly, these studies provided the first demonstration that angiogenesis could be disrupted by the pharmacological targeting of ADAM15 catalytic domain using selective AbIMPs thereby superceding previous efforts using non-selective chemical inhibitors. These proto-type ADAM15 AbIMPs represent the molecular blueprint for the development of a first-in-class anti-angiogenic therapeutic AbIMP candidate.
References
- Gomis-Ruth, FX. (2009). Catalytic Domain Architecture of Metzincin Metalloendoproteinases. J Biol. Chem. 284, 15353-15358.
- Murphy G. (2008). The ADAMs: Signalling scissors in the tumour microenvironment. Nature Reviews Cancer. 8, 929-941.
- Blobel CP. (2005). ADAMs: Key players in EGF signalling, development and disease. Nature Revs Mol. Cell. Biol. 6, 32-45.
- D.R. Edwards, M.M. Handsley, C.J. Pennington (2008). The ADAM metalloproteinases. Mol Aspects Med 29, 258-289.
- Lucas, N & Day, ML (2009). The role of the disintegrin metalloendoproteinase ADAM 15 in prostate cancer progression. J. Cell. Biochem. 106, 967-974.
- Horiuchi, K., G. Weskamp, L. Lum, H.P. Hammes, H. Cai, T.A. Brodie, T. Ludwig, R. Chiusaroli, R. Baron, K.T. Preissner, K. Manova, and C.P. Blobel. (2003). Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol. 23:5614-24.
- Xie B, Shen J, Dong A, Swaim M, Hackett SF, Wyder L, Worpenberg S, Barbieri S, Campochiaro PA. (2008). An ADAM15 amplification loop promotes vascular endothelial growth factor-induced ocular neovascularization. FASEB J.22(8):2775-83.
- Rahman, S., Lundkvist, A., Xu, C., Sumathipala, R., et al (2007). ADAM15 is essential for regulated angiogenesis and VEGF signalling to Akt via the proteolytic processing of uPAR. Heart 93, 1-4 (003).
- Salman Rahman, Yatin M Patel, Errol S Wijelath, Michael S Sobel (2008). Therapeutic potential of novel modulators of neovascularization. Future Cardiology, Vol. 4, No. 4, 409-426.
