AbIMP® is a novel discovery platform that utilises monoclonal antibodies as selective inhibitors of previously intractable metzincin protease targets thereby offering new possibilities for drug discovery and development.
The development of selective chemical inhibitors of metalloproteinases belonging to the metzincin superfamily remains a significant technical challenge despite decades of research and development predominantly focused on the matrix metalloproteinases (MMPs) (1). This has led to a paucity of clinically viable strategies to target these important proteases despite a plethora of data confirming their role in promoting disease..
The catalytic domain of metzincins is characterised by the presence of a shared structural scaffold and active site environment and although each subfamily has unique structural elements, very few genuinely selective inhibitors have been developed to date. Comprising of three alpha helices (αA, αB & αC) and five beta strands (sI-sV) which form a twisted β-sheet, the catalytic domain is spatially organised into N-terminal and a C-terminal sub-domains (Fig. 1) bifurcated by the active site cleft (catalytic cleft) which spans the entire width of the domain and harbours a catalytically active zinc ion (1,2). A further characteristic structural feature is the presence of a consensus zinc-binding motif HEXXHXXGXX(H/D) where X denotes any amino acid, which contains three zinc-binding histidines (or an aspartate at the third position) and a glutamate that acts as a general base/acid during catalysis. This motif is followed by an amino acid denoted “Z”, which varies but is characteristic of the metzincin subfamily, and a conserved structure called the “Met-turn” containing an invariant methionine “M”(Fig. 1).
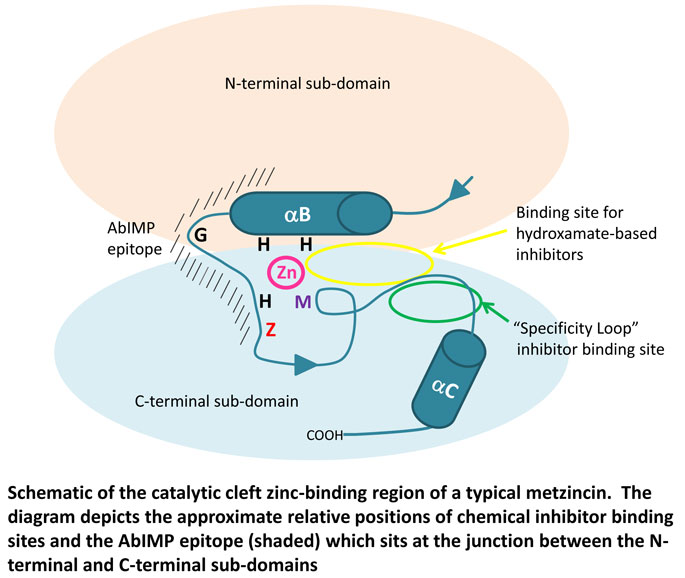
Fig. 1
AbIMP Technology Enables Selective Targeting of the ADAMs Gene Family
Vasgen has identified a strategy to potentially overcome the problem of selectively inhibiting individual members of the ADAMs family of sheddases which may extend to other metzincin subfamily enzymes. Using a targeted antibody approach, Vasgen has generated proto-type antibodies that display a high degree of selectivity for their ADAM targets and which inhibit ADAM-mediated ectodomain shedding in cell-based assays and ADAM-mediated cellular responses in vitro and in vivo. These antibodies are referred to as Antibody Inhibitor of Metalloproteinases or AbIMP
This exciting, new technology offers the potential to:
- Develop first-in-class therapeutic candidates that inhibit validated therapeutic ADAM targets for clinical evaluation.
- Pharmacologically validate ADAMs of therapeutic interest identified by genetic approaches.
- Measure the antigen/activity of a target ADAM of diagnostic/prognostic value.
Citations:
- Gomis-Ruth, FX. (2009). Catalytic Domain Architecture of Metzincin Metalloendoproteinases. J Biol. Chem. 284, 15353-15358.
- Murphy G. (2008). The ADAMs: Signalling scissors in the tumour microenvironment. Nature Reviews Cancer. 8, 929-941.
