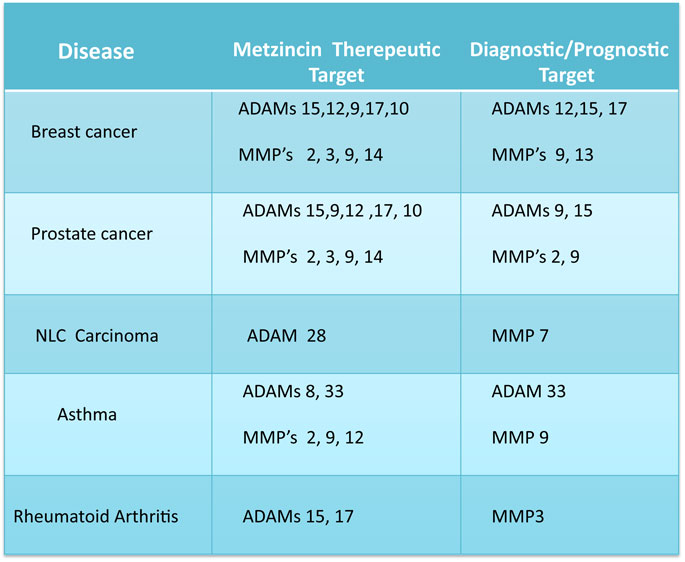
There is increasing evidence to indicate that the metzincin superfamily of metalloproteinases, in particular the ADAMs & MMPs, have important roles in the pathophysiology of many different diseases (refs 1, 2 & Table 1). However, progress with target validation and the development of therapeutic agents has been hampered by the lack of selective inhibitors of catalysis. Vasgen’s unique AbIMP™ technology represents a potential technological breakthrough that overcomes the problems encountered using non-selective chemical inhibitors as therapeutic candidates including off target effects and toxicity (2). In addition, generation of highly selective AbIMPs could potentially also be used to validate metzincins involved in disease progression in animal models and to develop novel, selective anti-metzincin therapeutic AbIMP candidates. This approach could lead to the generation of a new pipeline of first-in-class products.
Table 1 Potential Therapeutic and Diagnostic Metzincin Targets Associated with High Value Markets
Metzincin Diagnostic Market
The global market for In vitro diagnostics is a multibillion dollar industry dominated by the molecular diagnostics segment. The overall market is set to increase due to advancement of technology and the growing interest in personalised medicine and point of care diagnostics.
As shown in Table 1, several ADAMs and MMPs are potential candidate biomarkers of disease that have been described widely in the literature. In several ocular disorders and cancers, various ADAMs/MMPs (e.g. MMP9/sADAM 12) have been shown to be elevated in bodily fluids such as urine, plasma and ocular secretions opening the possibility of measurement of metalloproteinase activity for both diagnostic and prognostic purposes. Due to targeting the catalytic cleft, Vasgen’s AbIMP technology provides a potential mechanism to measure the specific activity of a target metalloproteinase in a complex biological sample without the need to immunocapture the metalloproteinase prior to activity measurement. Therefore, AbIMP technology can be potentially developed for both companion and point of care diagnostics for those validated disease biomarkers. A point of care device for diagnosing Dry Eye by measuring MMP 9 activity in patient tears has recently been brought to market. In addition, there is considerable interest in developing point of care devices measuring MMP levels/activity in diseases such as periodontal disease and processes such as wound healing.
Citations:
- Duffy MJ, McKiernan E, O’Donovan N, McGowan PM. (2009). The role of ADAMs in disease pathophysiology. Clin Chim Acta. 403 :31-6.
- Rodríguez D, Morrison CJ, Overall CM. (2009). Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 1803(1):39-54.
